- Hits: 2659
Phenylacetic acid production(PAA-precursor)
Phenylacetic acid (PAA) is the precursor needed for a high yield of penicillin G production according to the latest studies, so as per its importance, we are working on its local production.
Note on naming: alternative names for phenylacetic acid include:
- Benzeneacetic acid
- 2-Phenylacetic acid
- alpha-Toluic acid
- Phenylethanoic acid
Many methods are used for its production. Among them involves the acidic hydrolysis of benzyl cyanide, producing PAA and ammonium chloride as a byproduct (method 1).
Different methods of production:
Method 1 - Benzyl cyanide (REFERENCE)
*Reagents and Apparatus:
- Benzyl cyanide
- Hydrochloric acid
- Vacuum distillation apparatusSuction filtration apparatus
- Four necked flask (1000 mL)
- Reflux condenser
- Thermometer
- Dropping funnel
*Steps of production:
- Add 236.5 g (2 mol) benzyl cyanide to a 1000 mL four necked flask, with a reflux condenser, thermometer, and dropping funnel
- Add hydrochloric acid dropwise to the benzyl cyanide or dropwise adding the molten benzyl cyanide to hydrochloric acid, wherein the molar ratio of the hydrochloric acid to the benzyl cyanide is (1.2-5):1. The mass fraction of HCl is 15-37%.
- Heat the air tight reaction system to 95oC for 1-5 hours; and ending the reaction when the mass content of the benzyl cyanide in the reaction system is less than 5%
- Perform vacuum distillation for recovering the unreacted benzyl cyanide in the reaction system of the steps (1-3) until the mass content of the benzyl cyanide in the reaction system is less than 0.2%
- Add 700 mL water to the reaction system, and stir and mix
- Cool for crystallization to 30oC and perform suction filtration. Wash the obtained crystal with 300 mL warm water, and dry at 30-40oC under reduced pressure to obtain a phenylacetic acid product.
- You may repeat steps 4-6 to reclaim unreacted benzyl chloride on what remains after the distillation (the ammonium product, the excess hydrochloric acid)
According to the benzyl cyanide safety manual, this chemical is highly toxic. Therefore necessary safety precautions must be taken, such as personal protection equipment like gloves and safety goggles as well as appropriate ventilation and respirators.
Due to the dangers associated with this reagent, we are searching for other ways to synthesize PAA.
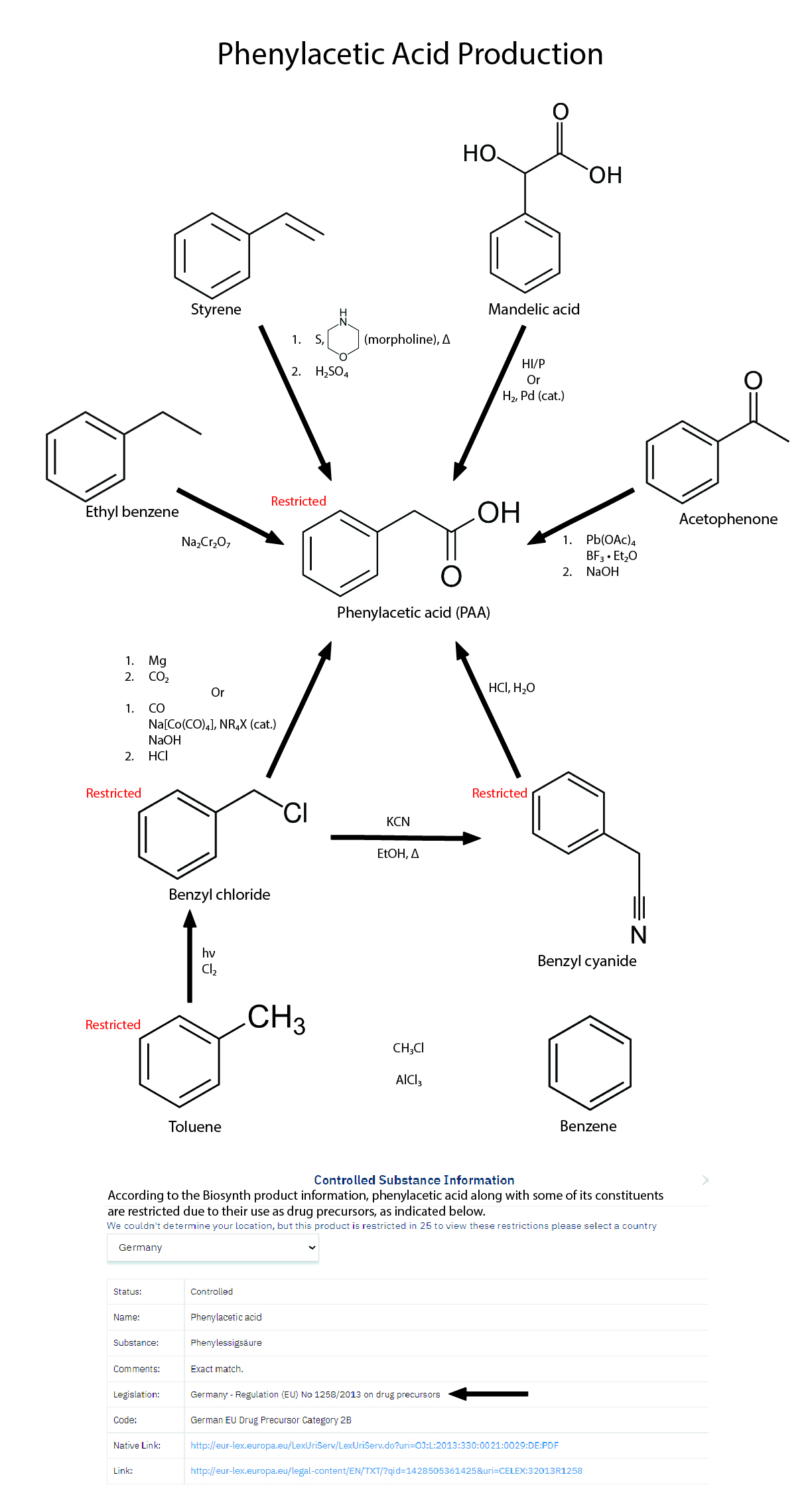
Method 2 - Mandelic Acid (A) (REFERENCE)

*Reagents and Apparatus:
- Mandelic acid
- Potassium iodide (KI)
- Red phosphorous
- Phosphoric acid
- Reflux apparatus
- Ether (for extraction)
- Separatory funnel
- Dilute NaHSO3
- Na2SO4
- Vacuum distillation apparatus
*Steps of production:
- 15 grams mandelic acid (0.1 mol), 2.07g KI (6.25% of theory), and 6g Red Phosphorous is dissolved in a solution of 70 ml phosphoric acid and 10 ml of water, and this solution is refluxed for six hours (bp of soln 144°C).
- After the reaction mixture has cooled down, a little water is added to dissolve precipitated inorganic salts.
- The solution is extracted with ether, and the pooled extracts washed with a little dilute NaHSO3, followed by water, and is then dried over Na2SO4.
- The ether is removed under diminished pressure and the residue is distilled (bp 138-139 @13mmHg). The yield is 12.5 grams (90%) of phenylacetic acid melting at 76°C. 3.2 grams of phosphorus could be recovered from the aqueous solution.
Method 3 - Mandelic Acid (B) (REFERENCE)
*Reagents and Apparatus:
- Mandelic acid
- Pd/BaSO4 catalyst
- Hydrogen gas
- Thin layer chromatography
- Autoclave with stirrer
*Steps of production:
A variety of methods are illustrated by this patent. Though they use p-hydroxymandelic acid hydrate in their examples, they state that mandelic acid is a suitable starting reagent, albeit the substituted derivatives are especially preferred. The highest yield and simplest method is described below:
- In an autoclave with a stirrer, a suspension of 0.5 g of Pd/BaSO4 (1% impregnation) in 50 ml of water is heated to 150° C. under a partial pressure of H2 of 5 barr and a solution of 20 g of mandelic acid in 50 ml of water is pumped in over a period of 2 hours.
- Hydrogenation is continued for 2 hours.
- The hot solution is filtered to separate the catalyst and the filtrate is concentrated to about 60 ml.
- On cooling to room temperature the main quantity of phenylacetic acid crystallizes over-night. A further amount thereof is obtained by concentration of the mother liquor. Total yield 13.6 g or 85% of the theoretical.
Method 4 - Acetophenone (REFERENCE)
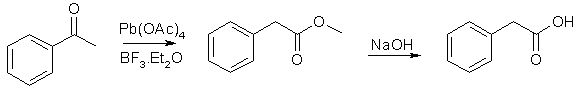
*Reagents and Apparatus:
- Acetophenone
- Methanol
- Boron trifluoride etherate
- Lead tetraacetate
- Benzene
- Sodium bicarbonate
- Sodium chloride
- Sodium sulfate
- Magnetic stirrer
- Sodium hydroxide
- Reflux apparatus
- Separatory funnel
- Vacuum distillation apparatus
*Steps of production:
Methyl phenylacetate
- 4.56g (38 mmol) acetophenone, 10ml methanol, 22g (150 mmol, 20ml) boron trifluoride etherate (BF3*Et2O) is added in one portion to a magnetically stirred suspension of 17.7g (40 mmol) lead tetraacetate in 100ml benzene.
- The reaction mixture is stirred at room temp for 5h.
- It is then diluted with 500ml cold water.
- Afterwards, it is extracted with 600ml benzene.
- Then, it is washed with 150ml satd. NaHCO3, 200ml satd. NaCl.
- Finally, it is dried over sodium sulfate and filtered.
- Evaporation of the solvent and vacuum distillation of the residue gave methyl phenylacetate in 86% yield, bp 94-96C/5mmHg.
Phenylacetic acid
- 2.4g (16 mmol) methyl phenylacetate and 10 ml 2M NaOH is refluxed for 2h.
- The free phenylacetic acid is precipitated by the addition of conc HCl (~3ml), in 92% yield, mp 76-76.5°C.
The Willgerodt Reaction can also be used to transform acetophenone (or styrene) to phenylacetic acid. See method 5.
Method 5 - Styrene (REFERENCE)
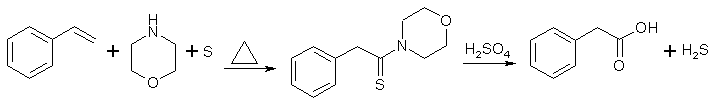
The Willgerodt-Kindler reaction with morpholine transforms acetophenones and styrenes to phenylacetic acids, through the intermediate phenylacetothiomorpholide, which is hydrolyzed in acid or basic solution to the desired acid.
*Reagents and Apparatus:
- Styrene
- Sulfur
- Morpholine
- Chloroform
- Hydrochloric acid
- Sulfuric acid
- Ether
- Caustic soda
- Reflux apparatus
- Vacuum distillation apparatus
- Separatory funnel
*Steps of production:
- A mixture of styrene (104 g, 1.00 mole), sulfur (80g, 2.5 moles) and morpholine (174 g) was refluxed two hours (internal temp rising to about 175°C).
- The cooled reaction mixture was taken up in chloroform (some sulfur remained undissolved) and the chloroform solution was washed successively with an equal volume of water, sufficient dilute hydrochloric acid to remove the excess morpholine, and finally with an equal volume of water.
- The solvent was removed under vacuum, and the residue of crude phenylthio-acetmorpholide was refluxed for ten hours with 1200 ml of 50% (by weight) sulfuric acid.
- The cooled hydrolysis mixture was extracted with three 500 ml portions of ether and the combined ethereal extract was washed with 100 ml of 12% caustic soda.
- The caustic wash was acidified with conc. HCl and then extracted three times with 300 ml portions of ether. Removal of the solvent from the combined ethereal extract gave 114g (84% yield) of nicely crystalline phenylacetic acid, m.p. 72-74°C.
One run in which the original reaction mixture was concentrated under vacuum on the steam cone and then hydrolyzed directly with 50% sulfuric acid gave only a 59% yield of phenylacetic acid, m.p. 71-74°C.
Note: One of the byproducts H2S is extremely flammable and highly toxic, so necessary precautions must be taken to ensure it does not escape as a gas. Such precautions include utilizing a fume hood.
Comparison (PPA Production Comparison.ppt)
In order to compare the various methods according to cost, feasibility, and safety, we organized the information in the attached PowerPoint and have decided to move forward with the method involving methylphenyl acetate. This is due to the fact that not only is it the most cost efficient and has significant yield (92%), but also none of the reagents have any significant toxicity. Furthermore, the procedure is straightforward, and the apparatus required has multiple uses and can be used in the future for different applications.
PAA production Poster:
(PAA production poster.pdf)17-2-2023

Hydrolysis of Penicillin G to PAA
In order to save time while waiting for the methyl phenylacetate to arrive, we decided to exploit the hydrolysis of penicillin G into PAA and 6-APA by the enzyme penicillin acylase in order to obtain PAA. This is due to the fact that we would like to test if PAA as the missing precursor is sufficient for the production of penicillin G by the Penicillium. Furthermore, conducting this study is helpful for the future as it shows how one may recycle the PAA produced as a byproduct of the reactions needed to result in ampicillin.

Image reference: Elnashar, 2010
Reference: CN102249891B - Method for recovering and purifying phenylacetic acid - Google Patents
Procedure for Trial 1
- React penicillin acylase with penicillin aqueous solution at 25oC and at a pH of 6.5 to obtain 6-APA and PAA.
- Extract with n-butyl acetate equal to ½ the volume of the reaction mixture. 6-APA will be concentrated within the aqueous phase whereas PAA will be concentrated within the organic phase.
- Take the PAA-butyl acetate, and add purified water of a volume that is ½ of the PAA-butyl acetate. Heat to 30oC and stir for 20 minutes. Then, wait another 20 minutes to allow the phases to separate. Retrieve the ester phase.
- Add another portion of purified water equal to ⅓ of the PAA-butyl acetate volume. Add 30% NaOH and adjust the pH until it reaches 12. Heat to 80oC, stirring for 30 minutes. Then leave it to stand for 20 minutes to allow the phases to separate, and take the aqueous phase. This removes the ester-soluble impurities.
- Add 30% hydrochloric acid to the PAA-Na liquid and adjust the pH to 2. Stir for two hours at 5oC. Use suction filtration to obtain the crystals.
The amount of solid obtained as a result of trial 1 was quite low, not enough to be weighed. Therefore, after consulting other papers, we adjusted some parameters such as the duration and pH and repeated the procedure.
Procedure for Trial 2
- Add penicillin acylase with 2% w/v penicillin aqueous solution in a 1:5 mass ratio of the enzyme to penicillin G. Stir vigorously to dissolve the penicillin G if used as a powder. Adjust the pH to 7.8. React at 37oC for 24 hr while stirring at 120 rpm.
- Adjust the pH to 6.5. In order to prevent undissolved reactants from clogging the apparatus, first filter the reaction solution. Extract with n-butyl acetate equal to ½ the volume of the reaction mixture. 6-APA will be concentrated within the aqueous phase whereas PAA will be concentrated within the organic phase.
- Take the PAA-butyl acetate, and add purified water of a volume that is ½ of the PAA-butyl acetate. Heat to 30oC and stir for 20 minutes. Then, wait another 20 minutes to allow the phases to separate. Retrieve the ester phase.
- Add another portion of purified water equal to ⅓ of the PAA-butyl acetate volume. Add 30% NaOH and adjust the pH until it reaches 12. Then add a volume of hydrogen peroxide equal to 0.5% of the total volume. Heat to 80oC, stirring for 30 minutes. Then leave it to stand for 20 minutes to allow the phases to separate, and take the aqueous phase. This removes the ester-soluble impurities.
- Add 30% hydrochloric acid to the PAA-Na liquid and adjust the pH to 2. Refrigerate overnight at 5oC. Use suction filtration to obtain the crystals.



Result :

The yield of The produced PAA was very low not usable for proceeding in the penicillin production plan.
