- Hits: 3209
Electrolysis System Concept/ System Design
System Design

check it here:
060419_1430_poster_multistack_electrolysis.pdf
Mechanical Design
Check the Electrolyser Mechanical Design Page for more info:
Hydrogen Storage:
check the full presentation: 050419_ElectrolysisOfWater.pptx
PCS Design
Check the Electrolyser Process Control System Design Page for more info:
Needed inormations for the design and the calculations :
The proposed spaces were 10.65, 9.20, 8.25, 7.25, 6.30, 6.05, 4.35, 4.15, and 3.40 millimetres. From the nine different analysed distances between electrodes, it can be said that the best performance was reached by one of the smallest distances proposed, 4.15 mm. When the same distance between electrodes was compared (the same and different distance between electrodes and separator), the one that had almost twice the distance (negative compartment) presented an increase in current density of approximately 33% with respect to that where both distances (from electrodes to separator) are the same. That indicates that the stichometry of the electrolysis reaction influenced the performance. [1]
L = A x R/ρ.
R = L x ρ / A = L / (A x K )
K =1/ρ .
U= R×I
current density i nalkaline electrolysis= 0,2-0,4 A/cm square
calculating the current and the voltage for the existing cells
the bigger cell in which the distance between the eectrodes is 3,6 cm
cell details :
The radius of the surface which touches the solution : 14,6 cm .
The distance between the electrode and the membrane : 3,6 cm .
We filled only 2/3 of the cell volume .
Temperature = 25 degree celsius
So R = (3,6 cm)/((446,21 cm square × 0,125 )) = 0,0645 Ω .
I = 178,484 Amperes
U = 11,52 Volt
the smaller cells in which the distance between the eectrodes is 1,8 cm
L = 1,8 cm
Inner Radius = 14,6 cm
So R =(1,8 cm)/((446,21 cm square × 0,125 )) = 0,0322 Ω
I = 178,484 Amperes
U =5,747 volt
calculating the current the voltage and the distance between electrodes of the multistage electrolyser cell :
L= A x ( 6)/(A ) x 0,3375 = 2,025 cm
I = 0,4 x the surface that touches the solution=0,4 x A=0,4×3,14×r×r
U = 2,4 Volt
So the distance betwee the two electrodes shall be 2,025 cm .
And from the text above we seen that it is better to divide this distance into 3 parts
2 parts on the hydrogen production site and one on the oxygene production .
That means 0,675 cm from the anode to the membrane and 1,35 cm from the caathode to the membrane .
References :
[1] : Straight-Parallel Electrodes and Variable Gap for Hydrogen and Oxygen Evolution Reactions
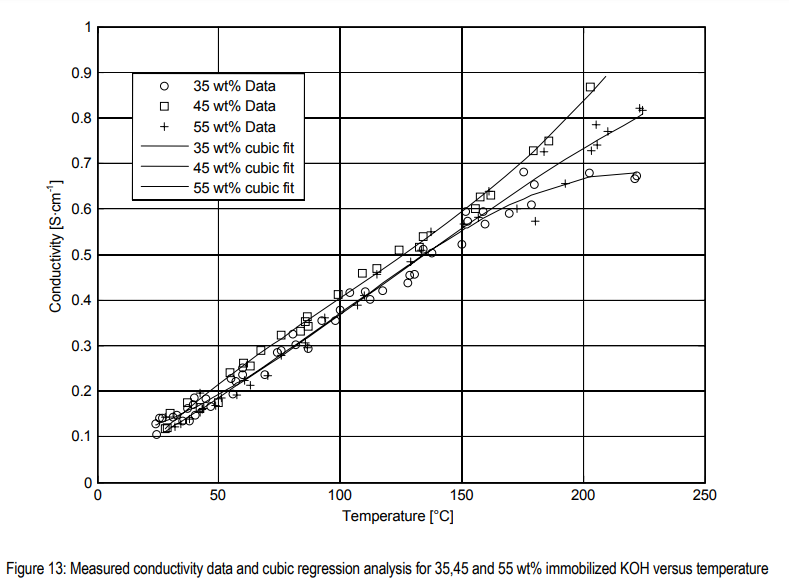
[2] : https://backend.orbit.dtu.dk/ws/portalfiles/portal/6368532/Electrical+conductivity+measurements.pdf
Related Sites/Items:
|
System Specification |
|
|
Mechanical Design |
|
|
Process Control System Spec./Design |
|
|
Mechanical Realization |
|
|
Process Control System Realization (PLC+GUI) |
|
|
System Test Specification |
|
|
System Testing |
