- Hits: 3289
Liquefication of Oxygen Prototype (ICPT-LOX) and Air Distillation
Distillation/Separation of oxygen, nitrogen and noble gases from liquid air
Design

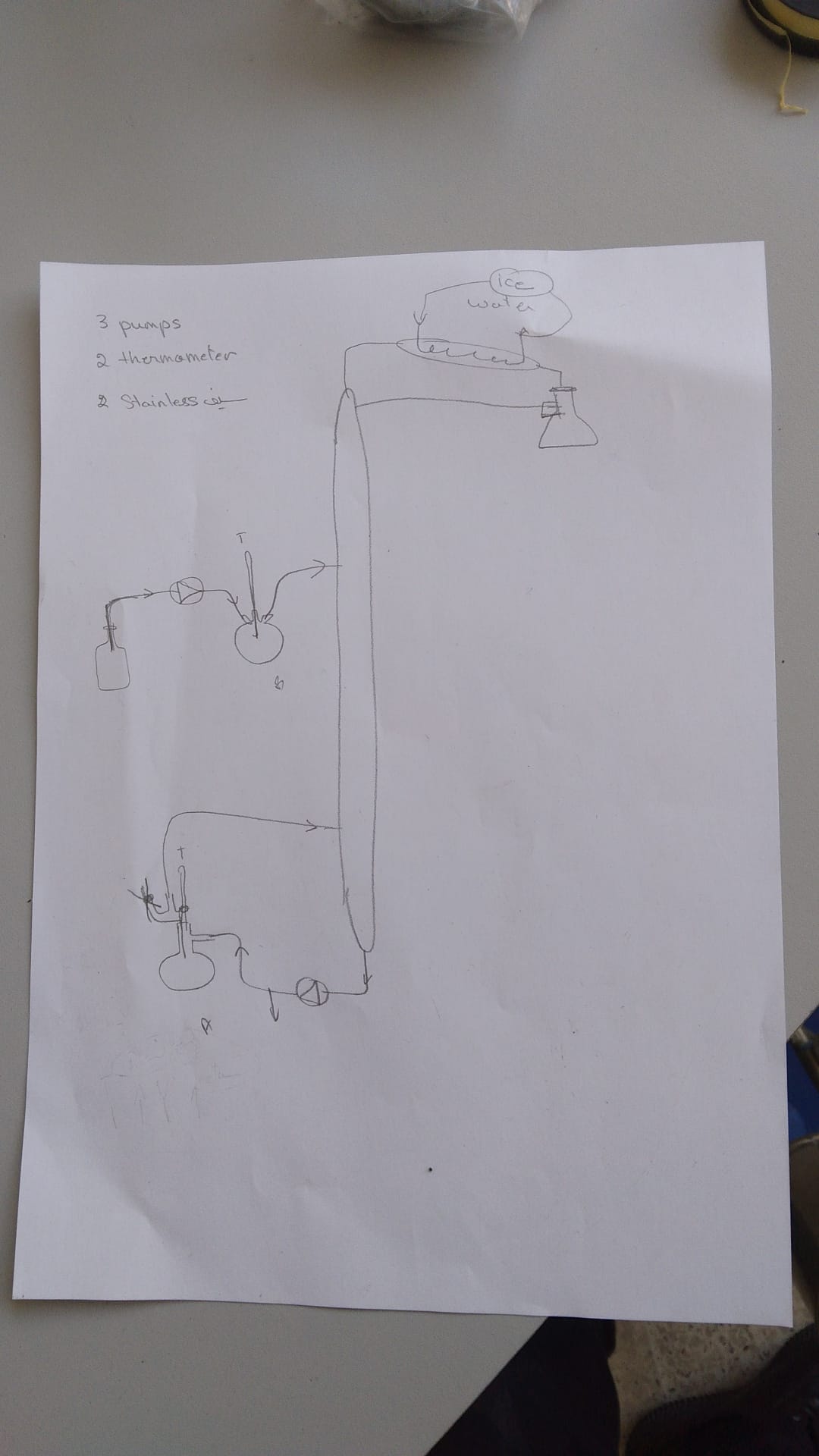
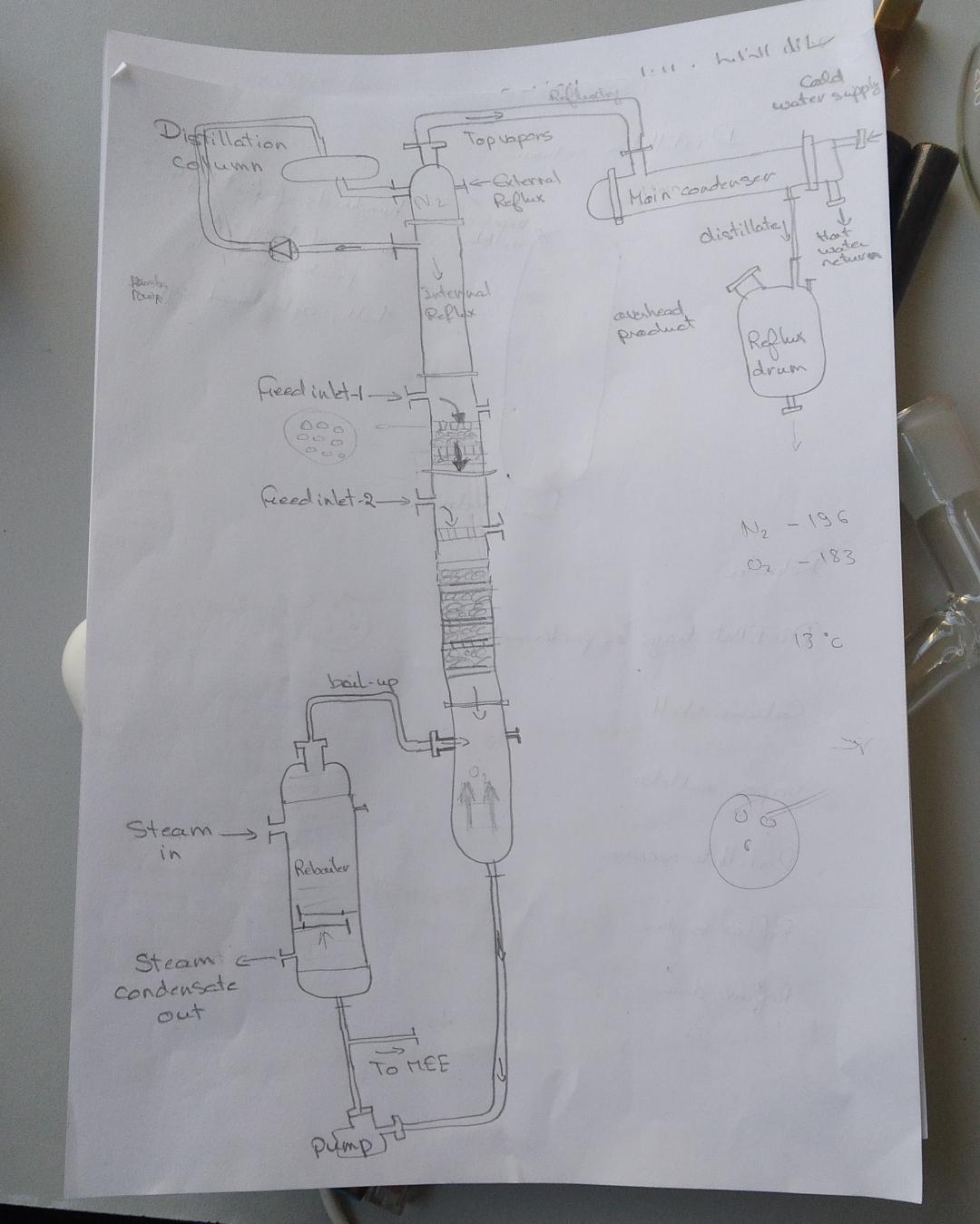

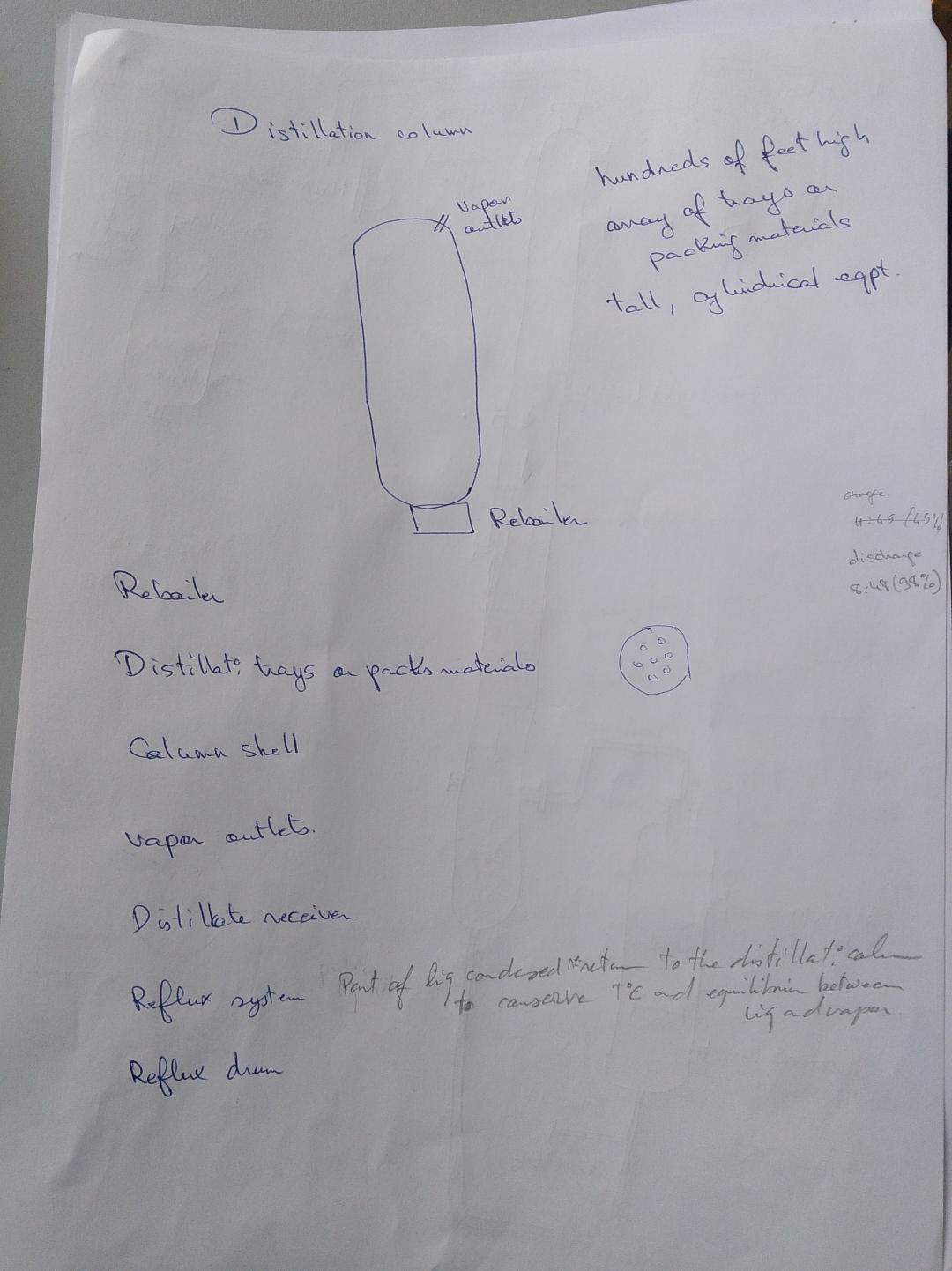

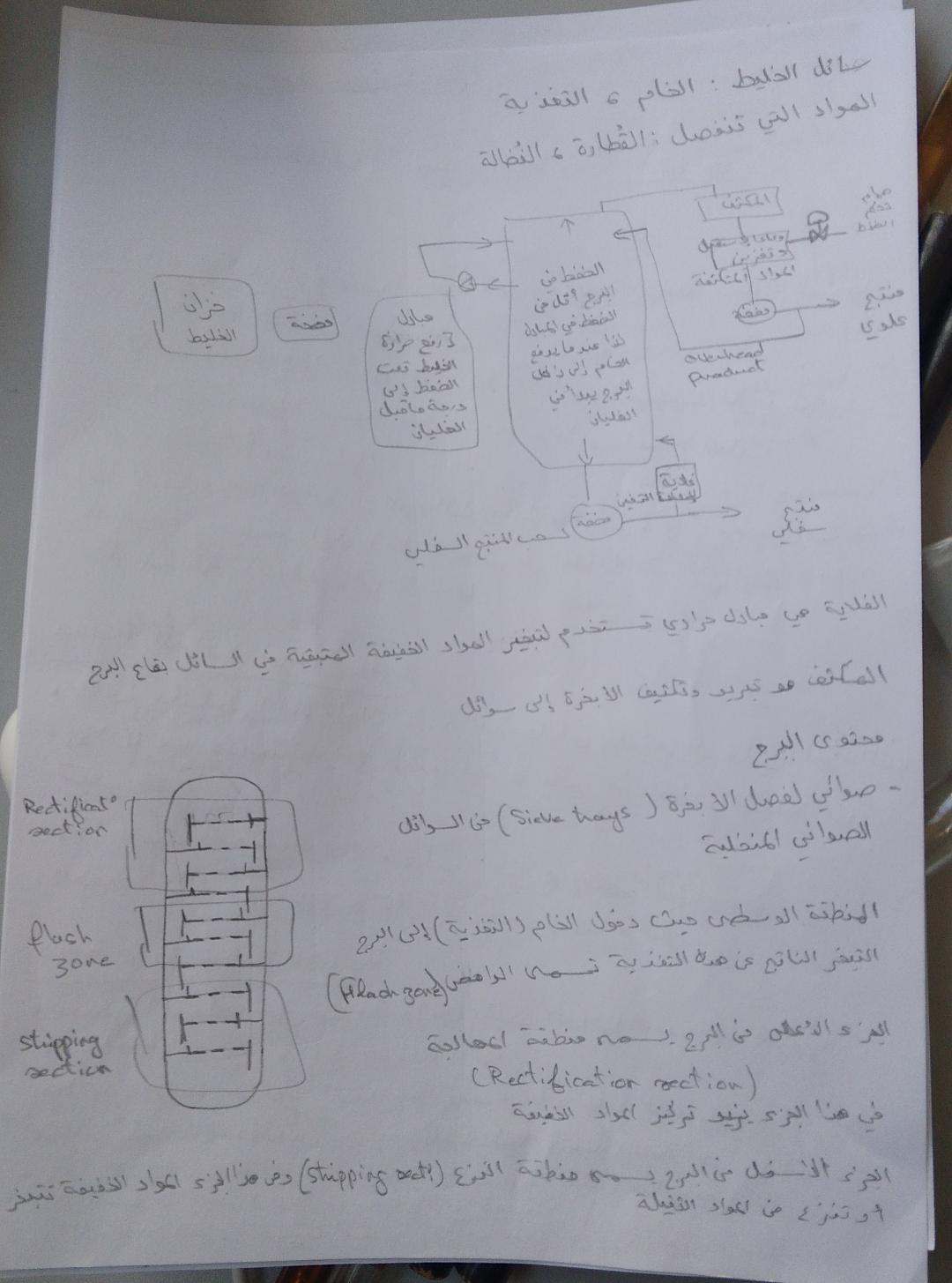


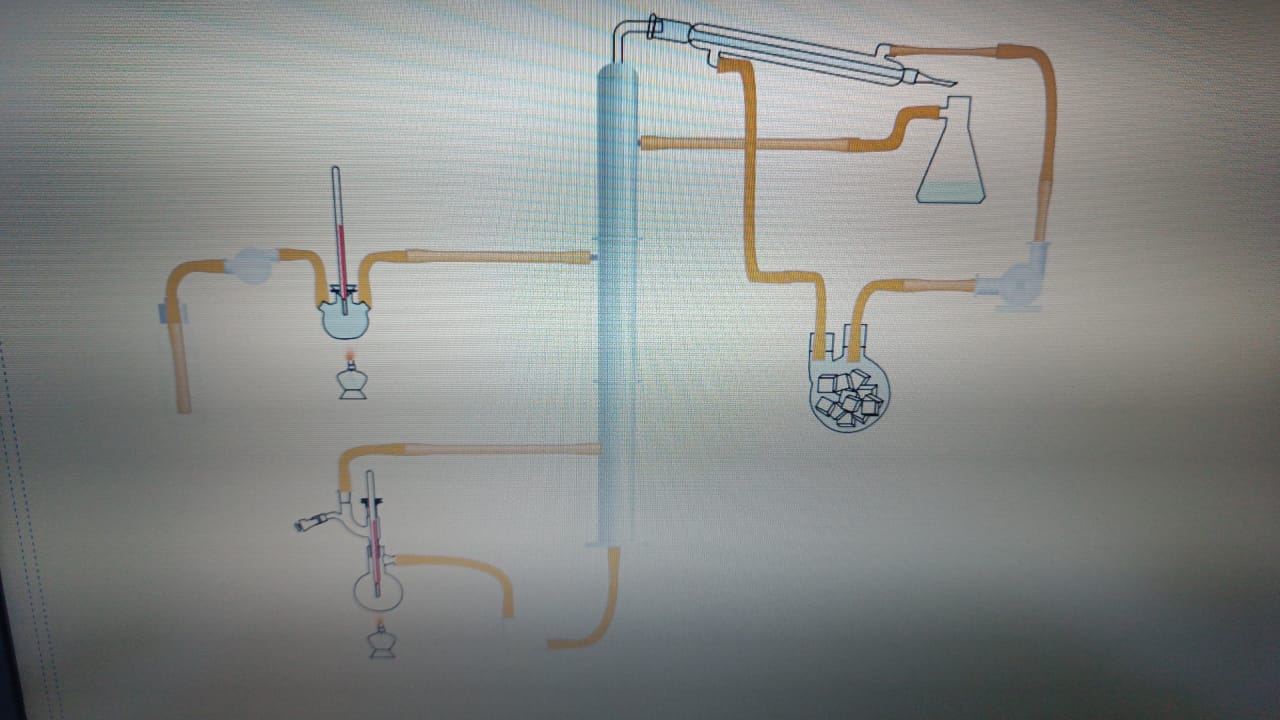
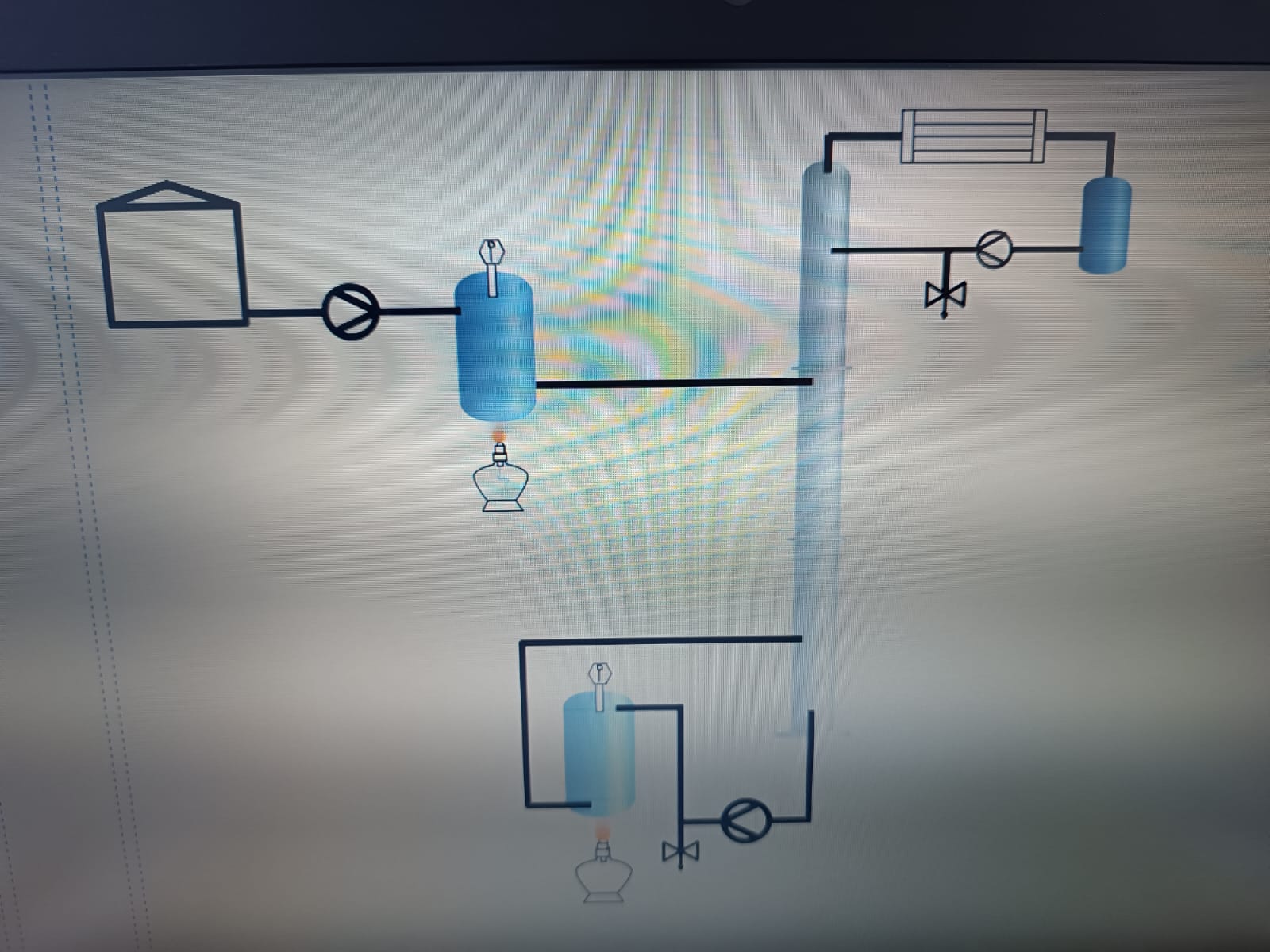


FreeCAD file 27.1.25:

https://aecenar.com/jdownloads/ICPT%20Intitute/270125DistillationTestStand.FCStd
Realization






28.1.25:



-------------------------------
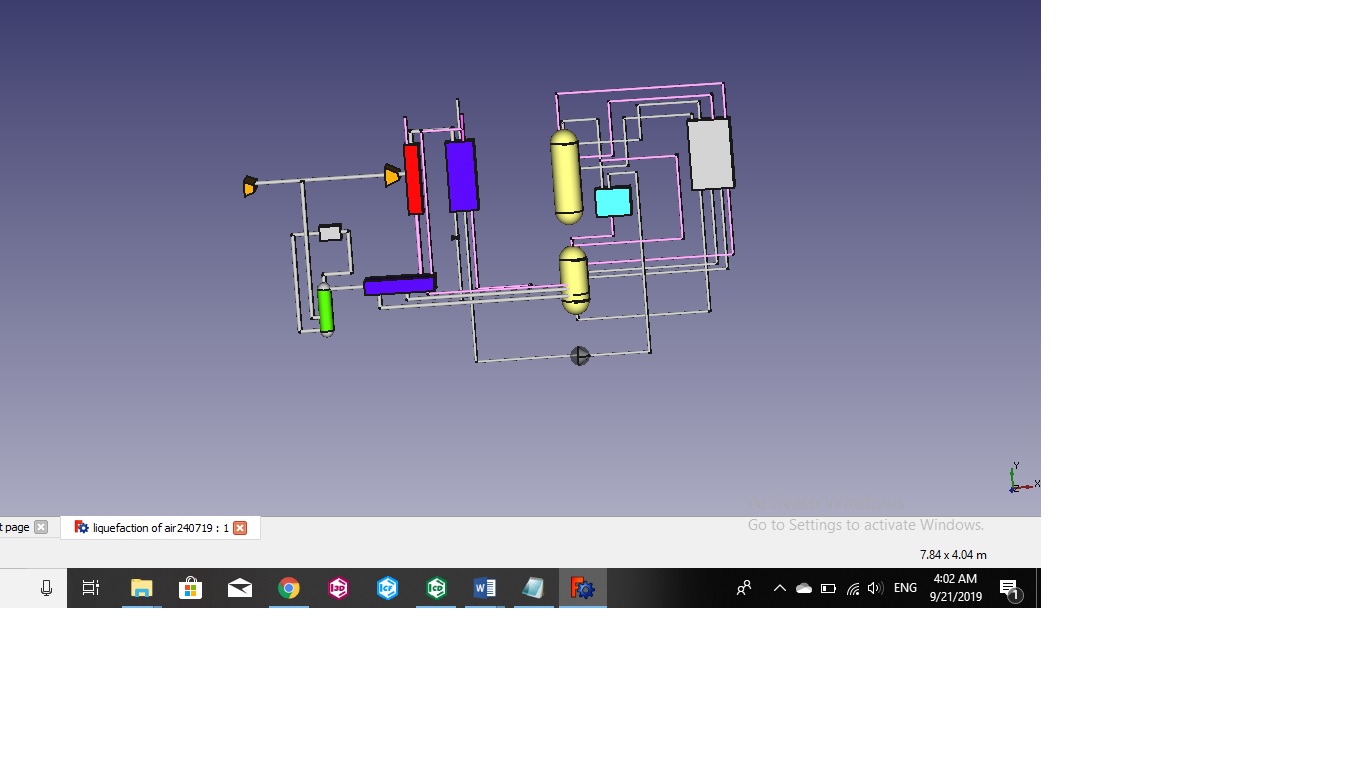
Liquefication of Air
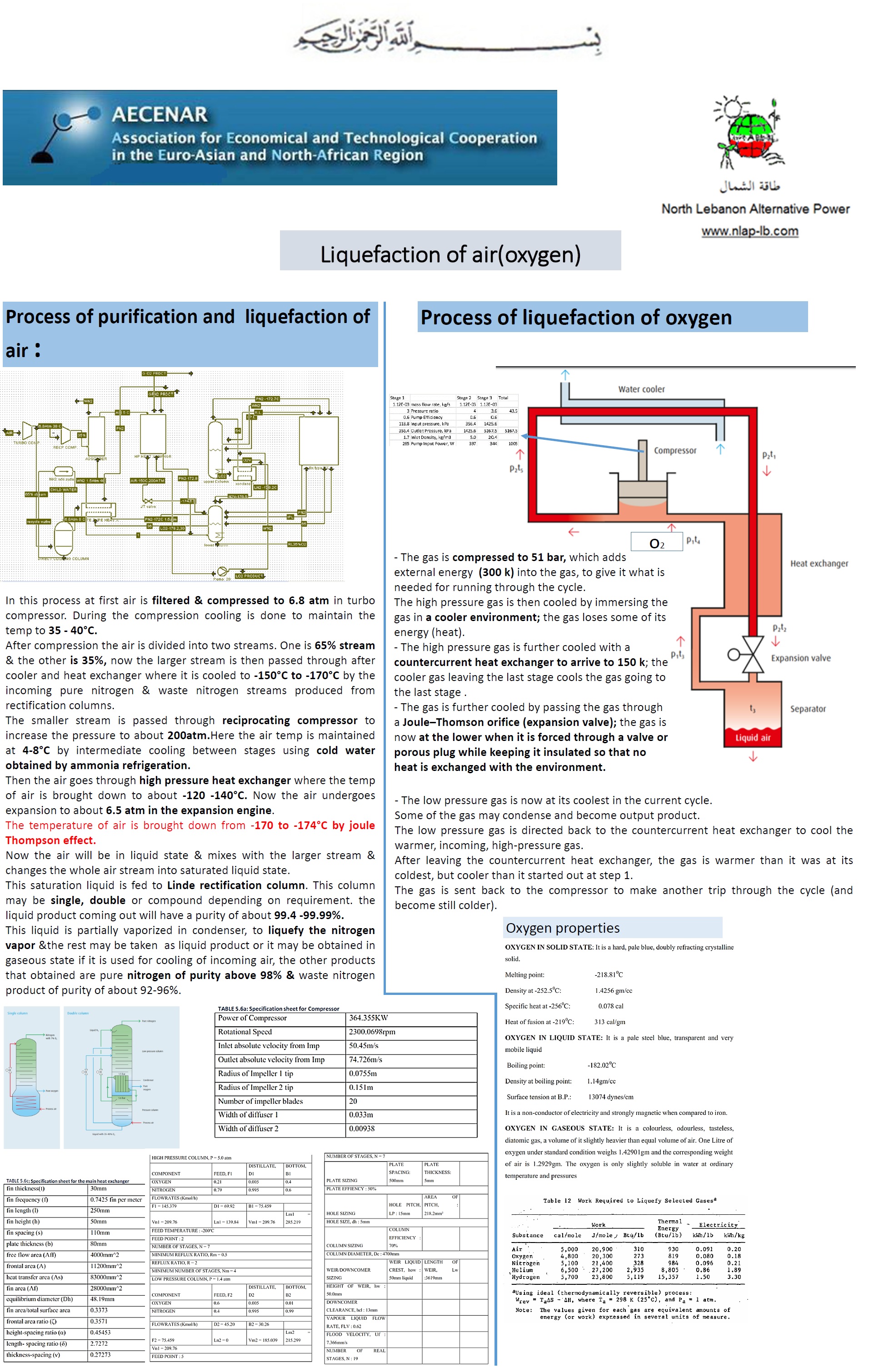
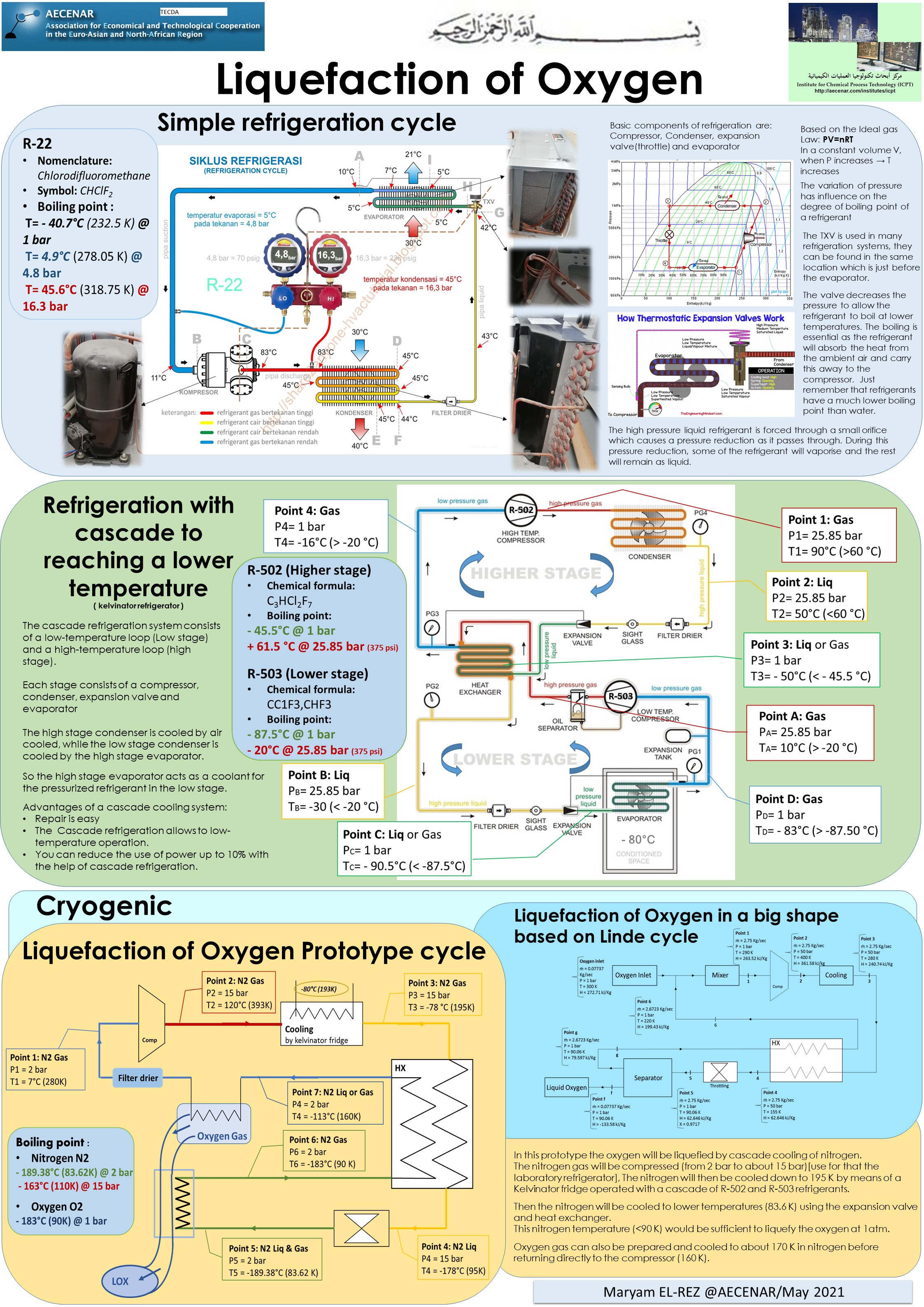
Liquefaction of Oxygen - Poster 1 (pdf)
Air Liquefication and Cryogenics - presentation (pptx)
Air Liquefication and Cryogenics - presentation (pdf)
Air Liquefication and Cryogenics - Report 2021, Part I: Basics (docx)
Air Liquefication and Cryogenics - Report 2021, Part I: Basics (pdf)
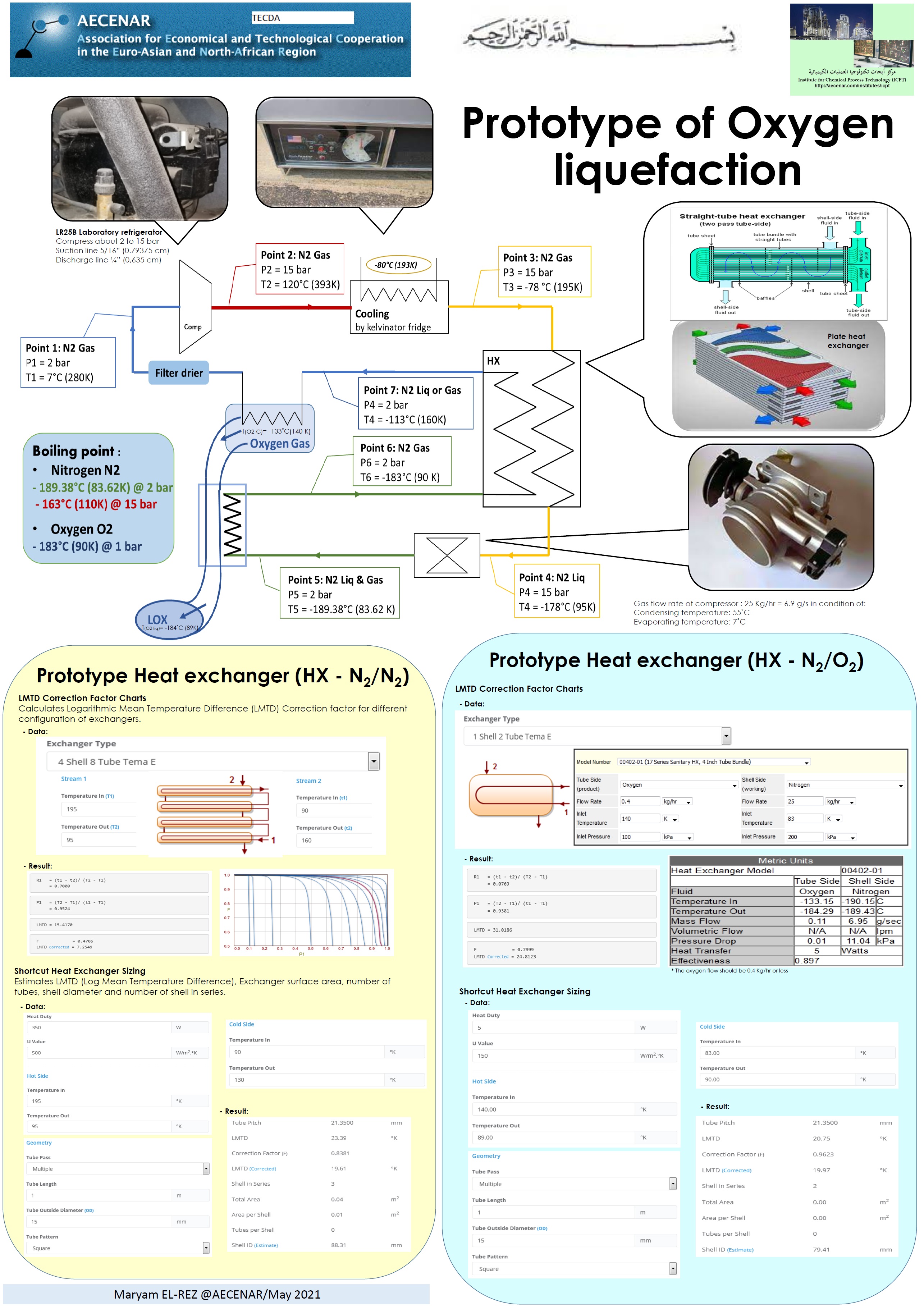
Liquefaction of Oxygen - Poster 2 (pdf)
|
Project |
System Concept, Mech. Design |
Mechanical Realization |
Process Control System (PLC+GUI) |
System Test / Commisioning |
|
ICPT-LOX |
|
|
|
Air Liquifecation - Realization

توصيلات مخصصة لمشروع تسييل الاوكسجين









Related Sites/Items:
|
System Specification |
|
|
Mechanical Design |
|
|
Process Control System Spec./Design |
|
|
Mechanical Realization |
|
|
Process Control System Realization (PLC+GUI) |
|
|
System Test Specification |
|
|
System Testing |
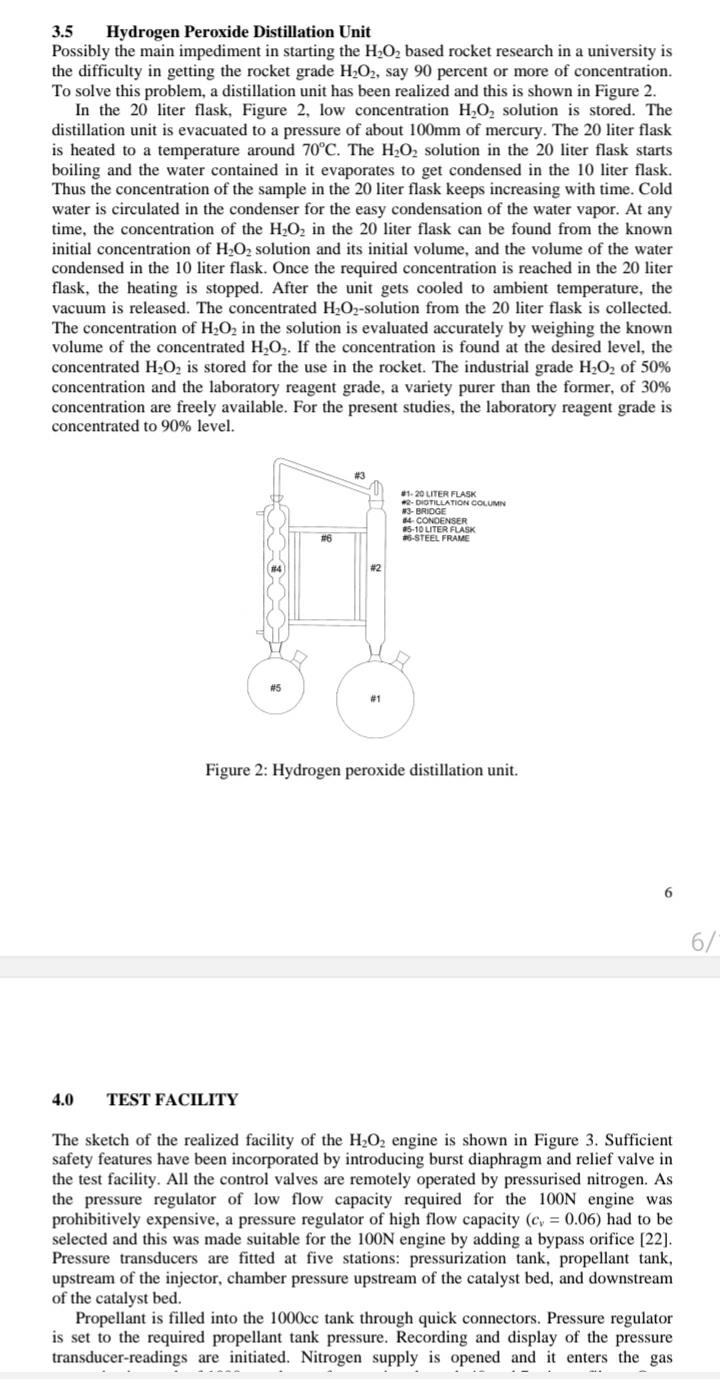
Example for Distillation: H2O2 50% to 90% upgrading
Hydrogen peroxide is often referred to as water with one more oxygen atom. It is acidic in nature, and PH is about 4.5. It is a 100 per cent degradable compound.
Hydrogen Peroxide Chemical Formula H2O2
Molecular Weight/Molar Mass 4.0147 g/mol
Density 1.05 g/cm3
Boiling Point 150.2 °C
Melting Point -0.43 °C
Properties of Hydrogen Peroxide
Physical Properties
• In the pure state, hydrogen peroxide is an almost colourless (very pale blue) liquid.
• It melts at 272.4 K and has a boiling point of 423 K (extrapolated).
• It is miscible in water in all proportions and forms hydrates.
Chemical Properties
Hydrogen peroxide in both acidic and basic mediums acts as an oxidising as well as a reducing agent. The following reactions will give a clear picture:
Why Is Hydrogen Peroxide Stored in Plastic Containers?
Hydrogen peroxide decomposes when exposed to sunlight, this process is catalysed by traces of alkali metals. Therefore, H2O2 is stored in wax-lined glass or plastic containers and kept in the dark.
It should also be kept away from dust particles because dust can induce explosive decomposition of this compound.
==============================
Boiling Point Of Hydrogen Peroxide
The boiling point of hydrogen peroxide is 150.2 °C (302.3 °F) at atmospheric pressure (1 atm, which converts to 14.6 PSI). This is approximately 50 °C higher than the boiling point of water, which is 100 °C. This chemical undergoes thermal decomposition (which is decomposition caused by heat) and boils explosively at this temperature, so it is not advisable.
Heat Capacity Of Hydrogen Peroxide
The specific heat capacity of liquid hydrogen peroxide is 2.619 J/(g-K), and in gas form, it is 1.267 J/(g-K). This (specific heat) refers to the amount of energy required to raise the temperature of hydrogen peroxide, not the latent heat. Latent heat refers to heat that results in the chemical's expansion. The latent heat of vaporization for hydrogen peroxide is 542 BTU/pound.
This means it takes 542 BTU of heat to convert 1 pound of H2O2 into its gas phase (convert it into a gas).
Density Of Hydrogen Peroxide
The density of hydrogen peroxide is 1.11 g/cm3 (1.11 grams per cubic centimeter), which means that a cubic centimeter of H2O2 weighs 1.11 grams.
============
Purifying hydrogen peroxide from water is challenging due to several factors:
• Azeotrope Formation: Water and hydrogen peroxide form an azeotrope at a specific concentration. This means that at this point, the vapor phase has the same composition as the liquid phase, making further separation by simple distillation impossible.
• Thermal Decomposition: Hydrogen peroxide is thermally unstable and decomposes into water and oxygen at elevated temperatures, making traditional distillation methods difficult.
Methods for Concentration (but not complete purification):
• Vacuum Distillation: Lowering the pressure reduces the boiling points of both water and hydrogen peroxide, allowing distillation at lower temperatures and minimizing decomposition.
• Extractive Distillation: Using a third component (entrainer) to break the azeotrope.