- Hits: 1500
Raw Materials production
Acetic Anhydride production
I- From Acetic Acid
Acetic anhydride is produced from glacial acetic acid via ketene (CAS no 463-51-4). Acetic acid is vaporized and fed together with a catalyst to a cracking furnace operating under vacuum where ketene is produced together with water at high temperature. The reaction mixture is rapidly cooled to avoid the reversing of the reaction. The condensed dilute acetic acid is separated and the gases then pass through two absorption towers in which they are scrubbed by acetic acid/ acetic anhydride of various concentrations.
Glacial acetic acid is added to the second absorption tower. Ketene is reacting with acetic acid to form acetic anhydride. In following washing towers the gases are further scrubbed before released to the atmosphere. In a distillation column the crude acetic anhydride is distilled and recovered as the bottom product. The top product is acetic acid which is recycled to the process.
Ref : IBI Chematur
II- production of acetic anhydride from carbon monoxide, acetic acid and methanol.
Acetic anhydride and hydrogen iodide can be prepared by thereaction of methyl iodide, methanol or acetic acid, and carbon monoxide under carbonylation conditions using a catalyst comprising a Group VIII metal and a Lewis base promoter. By reacting the hydrogen iodide with methanol to produce methyl iodide and recycling the methyl iodide an integrated process for the production of acetic anhydride can be devised which uses only methanol or methanol and acetic acid as organic feedstocks. The process has the advantage over existing technology that methyl iodide and water produced by the reaction of hydrogen iodide and methanol can be separated without distillation.
acetic anhydride and hydrogen iodide. The two products are readily separated e.g. by distillation, and both may be recovered pure. It is a particularily important feature of this invention that the hydrogen iodide is recoverable since it is able to react quantitatively with methanol to form methyl iodide and water
is in some respects similar to the esterification reaction used in the prior art to product methyl acetate
since both reactions require water removal from either methyl iodide or methyl acetate before carbonylation can occur. However, unlike the methyl acetate and water mixture which forms a single phase separable only by distillation, methyl iodide and water mixtures from two separate phases, easily separable by low energy methods such as decanting. Furthermore, although small amounts of methyl iodide can dissolve in water no azeotrope is formed and therefore separation by distillation is easy.
1) in a carbonylation stage a mixture of methyl iodide and acetic acid is reacted, under carbonylation conditions, with carbon monoxide in the presence of the catalyst described previously to form acetic anhydride and hydrogen iodide
2) in a separation stage the hydrogen iodide and acetic anhydride are separated and
3) in an iodination stage the hydrogen iodide and methanol are reacted to produce methyl iodide and water, the methyl iodide being subsequently separated from the water and recycled to stage (1).
The product stream withdrawn from stage (1) can consist exclusively of acetic anhydride and hydrogen iodide but more generally consists of a mixture of acetic anhydride, hydrogen iodide and unreacted reactants. In this case, stage (2) can consist of several steps which allow
(a) the unreacted reactants to be isolated and recycled to stage (1);
(b) the hydrogen iodide to be isolated and fed to stage (3) and
(c) the acetic anhydride to be recovered for sale or secondary uses.
Example 1
Example 2
Example 3
A 100 ml Hastelloy 'B2' autoclave was charged with 38.6 g of methyl iodide, 30.2 g acetic acid, 5.0 g triphenylphosphine and 0.200 g rhodium acetate dimer and pressurized with carbon monoxide to 65 bar. The vessel was then heated at 150°C during 3 hours with stirring. After cooling, g.c. analysis of the reaction mixture showed it to contain 3.1 g of acetic anhydride, hydrogen iodide and 31.2 g of acetic acid.
Claims
(1) a Group VIII noble metal preferably rhodium
(2) a Lewis base promoter.
1) in a carbonylation stage a mixture of (a) methyl iodide and (b) acetic acid and/or methanol is reacted under carbonylation conditions with carbon monoxide in the presence of the carbonylation catalyst claimed in Claim 1 to form acetic anhydride and hydrogen iodide,
2) in a separation stage the hydrogen iodide and acetic anhydride are separated, and
3) in an iodination stage the hydrogen iodide and methanol are reacted to produce methyl iodide and water, the methyl iodide being subsequently separated from the water and recycled to stage (1).
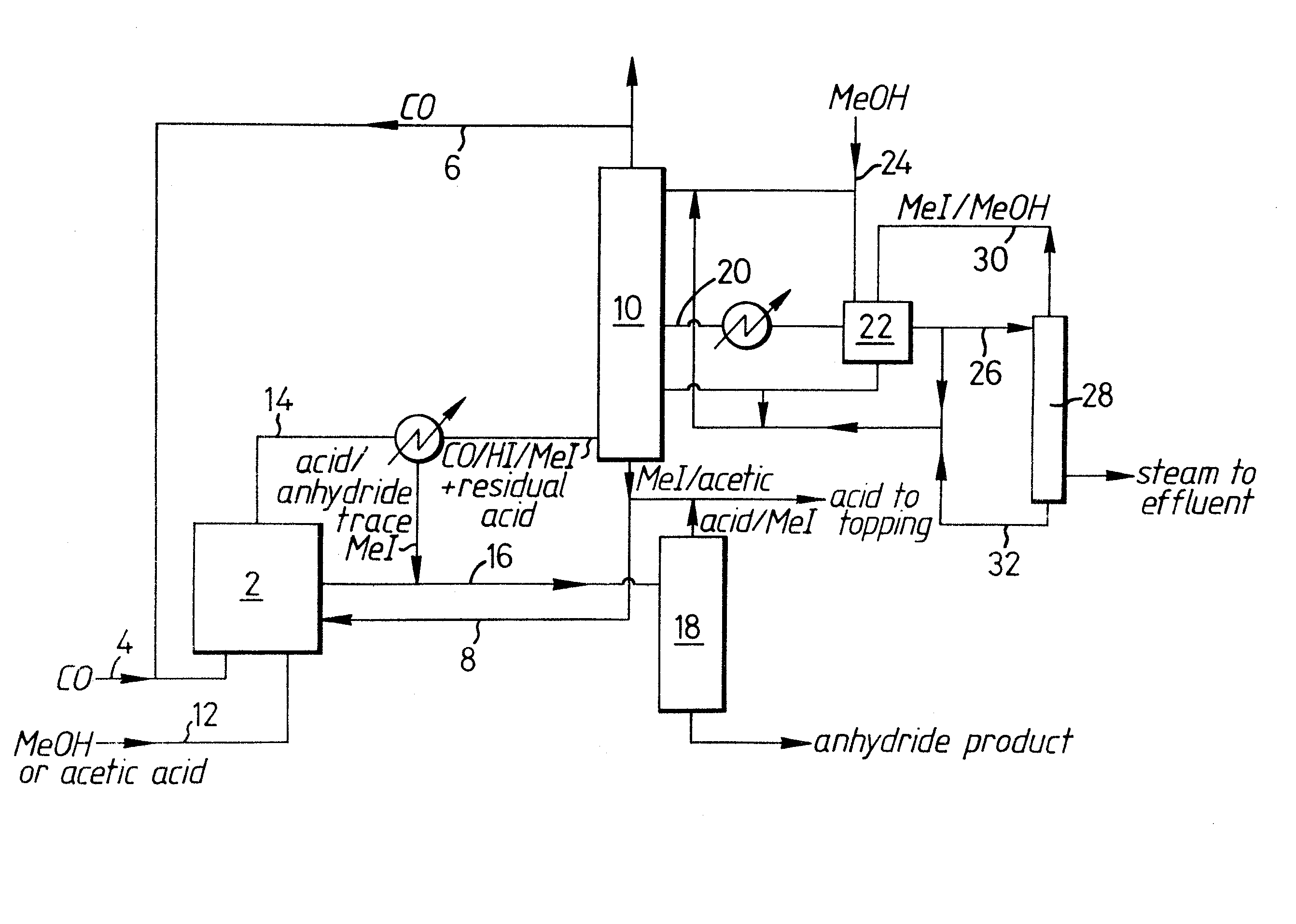
Ref : EPO - European publication server
Process for the production of acetic anhydride and acetic acid
A process for the production of acetic anhydride with or without the net co-production of acetic acid from methanol and carbon monoxide involves the following steps
1) reacting methanol with recycle acetic acid in an esterification step to form an esterification product containing predominantly methyl acetate, water and optionally unreacted methanol,
2) removing part of the water from the esterification product,
3) reacting the esterificaion product still containing water with carbon monoxide in a carbonylation step in the presence as catalyst of free or combined metallic carbonylation catalyst and as promoter of free or combined halogen to form a carbonylation product containing acetic acid and acetic anhydride,
4) separating the carbonylation product by fractional distillation into a low boiling fraction containing carbonylation feed and volatile carbonylation promoter components, acetic acid and acetic anhydride fractions, and a higher boiling fraction containing carbonylation catalyst components,
5) recycling the low boiling fraction containing carbonylation feed and carbonylation promoter components and the higher boiling fraction containing carbonylation catalyst components to the carbonylation step and,
6) recycling at least part of the acetic acid fraction to the esterification step.
1) reacting methanol with recycle acetic acid in an esterification step to form an esterification product containing predominantly methyl acetate, water and optionally unreacted methanol,
2) removing part of the water from the esterification product,
3) reacting the esterification product still containing water with carbon monoxide in a carbonylation step in the presence as catalyst of free or combined metallic carbonylation catalyst and as promoter of free or combined halogen to form a carbonylation product containing acetic acid and acetic anhydride,
4) separating the carbonylation product by fractional distillation into a low boiling fraction containing carbonylation feed and volatile carbonylation promoter components, acetic acid and acetic anhydride fractions, and a higher boiling fraction containing carbonylation catalyst components,
5) recycling the low boiling fraction containing carbonylation feed and carbonylation promoter components and the higher boiling fraction containing carbonylation catalyst components to the carbonylation step and,
6) recycling at least part of the acetic acid fraction to the esterification step.
With regard to the individual steps, in the esterification step (1) methanol is reacted with recycle acetic acid to form esterification product containing methyl acetate, water and unreacted methanol. In a preferred embodiment recycle acetic acid forms substantially the entire acetic acid in the feed to the esterification. It is an advantage of the present invention that neither the methanol nor the recycle acetic acid need be essentially pure. Thus the methanol may be contaminated with water, for example, and the recycle acetic acid may contain, for example, water, methyl acetate, acetic anhydride, and carbonylation promoter components, such as alkyl halides, eg methyl iodide. Though the esterification step may, if desired, be effected in the absence of a catalyst, it is preferred to employ an esterification catalyst, which may be any of the esterification catalysts conventionally employed. Suitable esterification catalysts include mineral acids such as sulphuric acid and organic acids such as toluene-para-sulphonic acid. Alternatively, other esterification catalysts, such as acid ion-exchange resins, may be employed if so desired. The esterification catalyst may suitably be present in an amount from 0.1 to 10%, preferably from 2 to 6X by weight of the reaction mixture.
(1) Esterification
(2) Carbonylation
(3) Hydration
The overall equation is therefore:
It will be seen from the last equation that, provided some water is removed, then acetic anhydride will be produced.
The greater the proportion of water removed then the higher will be the yield of acetic anhydride rather than acetic acid. Since the object of the present invention is to coproduce acetic acid and acetic anhydride it is important to remove some, but not all, of the water. Preferably water is removed to reduce the water content to below 14% w/w or the equivalent amount of water and methanol for example to below 12% w/w based on the weight of ester + water + alcohol if present. Preferably however at least 6X of water (as water) is present more preferably at least 7% w/w based on the amount of ester + water + alcohol.
In step (3), the esterification product containing predominantly methyl acetate, optionally unreacted methanol, and still containing water is reacted with carbon monoxide in a carbonylation step in the presence as catalyst of free or combined metallic carbonylation catalyst and as promoter free or combined halogen to form a carbonylation product containing acetic acid and acetic anhydride. Any of the known metallic carbonylation catalysts may be employed. Suitable metals include the metals of Group VIII of the Periodic Table of the elements, of which the noble metals iridium, osmium, platinum, palladium, rhodium and ruthenium are preferred. Particularly preferred is rhodium. It is preferred to employ the metal in the form of a soluble compound _such as a salt, eg a rhodium halide, or a complex of the metal, eg a carbonyl complex. As promoter there is used a halogen in free or combined form. Suitable forms include elementary halogen, a hydrogen halide, an inorganic salt, such as for example sodium halide, potassium halide or cobalt halide and quaternary ammonium or phosphonium halide, such as for example tetramethylammonium halide. Particularly preferred as promoters are organo-halides, such as alkyl, eg methyl iodide, or aryl halides. Methyl iodide is particularly preferred. Suitable reactants and conditions of operation are more fully described in the aforesaid UK Patents Nos. 1468940 and 1,523,346. It is particularly preferred to employ co-promoters. Suitable co-promoters include the metals in free or combined form such, as zirconium and those described in the aforesaid UK Patent No. 1468940, eg lithium, magnesium, calcium, titanium, chromium, iron, nickel and aluminium, alkyl or aryl 7 phosphines and organic nitrogen compounds as described in the aforesaid UK patent No. 1523346 and either heterocyclic aromatic compounds in which at least one heteroatom is a quaternary nitrogen atom as described in European Patent No. 8396 or the precursors of heterocyclic aromatic compounds in which at least one heteroatom is a quaternary-nitrogen atom, such as for example N-methyl imidazole. Other suitable promoter sytems are described for example in UK Patent No. 1538783 and UK published applications Nos 2059794, 2067557 and European published application No. 26280.
In one embodiment the carbonylation product from step (3) is separated in a first separation step by fractional distillation into an overhead fraction containing carbonylation feed and carbonylation promoter components, an intermediate fraction containing acetic acid and acetic anhydride, and a lower fraction containing carbonylation catalyst components, the overhead fraction and the lower fraction are recycled to the carbonylation step, the intermediate fraction is separated in a second separation step by fractional distillation into an acetic acid product fraction and an acetic anhydride product fraction, and part or all of the acetic acid product fraction is recycled to the esterification step.
In step (4), the carbonylation product is separated by fractional distillation into a low boiling fraction containing carbonylation feed and carbonylation promoter components, acetic acid and acetic anhydride fractions, and a higher boiling fraction containing carbonylation catalyst components.
In a preferred embodiment of steps (4),(5) and (6) the carbonylation product is separated in a first separation step by fractional distillation into an overhead fraction containing carbonylation feed and carbonylation promoter components, an intermediate fraction containing acetic acid and acetic anhydride, and a lower fraction containing carbonylation catalyst componerts, the overhead fraction and the lower fraction are recycled to the carbonylation step, the intermediate fraction is separated in a second separation step by fractional distillation into an acetic ?cid product fraction and an acetic anhydride product fraction, and at east part of the acetic acid product fraction is recycled to the esterification step.
Step (4) may suitably be effected by passing the carbonylation product to a tlash zone from which there is recovered a liquid product which is predominantly acetic acid and anhydride but also contains the metallic catalyst components and any co-promoter present in the product and a volatile product containing acetic acid, acetic anhydride and volatile carbonylation promoter and feed components, eg alkyl halides such as methyl iodide and methyl acetate. Optionally, a partial pressure of carbon monoxide and/or hydrogen may be applied in the flash zone to assist in maintaining catalyst activity. Further details may be found in UK patent application publication No. 2037276A which is hereby incorporated by reference. The volatile fraction may suitably be fed to a distillation column wherein volatile carbonylation promoter and feed components are removed overhead optionally together with some acetic acid and anhydride, and recycled to the carbonylation zone, a liquid sidestream comprising acetic acid and acetic anhydride is taken from an intermediate point and a liquid residue containing involatile carbonylation catalyst components carried over from the flash stage is removed from the base for recycle to the carbonylation zone with the liquid product from the flash zone. If the catalyst carry over from the flash stage is acceptable, the acetic acid/anhydride fraction may be taken from the base of the distillation column containing less low boilding impurities. The side or base stream containing acetic acid and acetic anhydride is then fed to a further distillation column from which pure acetic anhydride is removed as base product, and impure acetic acid is removed as overhead product. The impure acetic acid overhead fraction, which may contain some acetic anhydride and small amounts of methyl acetate and methyl iodide depending on whether the acetic acid/anhydride stream was taken as a side stream or a base product from the previous distillation column is then recycled directly to the esterification step. A part of this acetic acid stream may if desired be removed and purified in a further column to produce pure acetic acid.
The process of the present invention is further illustrated with reference to the accompanying drawing which is a simplified flow diagram of a process for the manufacture of acetic anhydride and acetic acid from methanol and carbon monoxide in a series of integrated esterification, carbonylation, and separation steps.
In this process the esterification step is carried out continuously in the kettle of a single distillation column 1, with n-butyl acetate as internal entrainer and side decantation to remove water by line 4. Internal entrainer is recycled to the column. Recycle acetic acid is fed to the kettle by line 2, together with an at least equimolar quantity of methanol by line 3, and a mixture of methyl acetate with some water and unreacted methanol is removed as head product by line 5 and passed directly to the carbonylation reactor 6. In the carbonylation reactor 6 the esterification product is reacted with carbon monoxide, fed to the reactor by line 7, in the presence of a rhodium carbonylation catalyst recycled by line 13, a promoter of methyl iodide recycled by line 12, and a co-promoter of N-methyl imidazole recycled as quaternary ammonium salt by line 13. The initial catalyst components are charged by line 8, which is also used for any subsequent make-up. The product of the carbonylation reaction consisting of predominantly acetic anhydride, acetic acid, unreacted methyl acetate and some methyl iodide is passed by line 10 to the separation zone 11 in which it is separated into a low boiling overhead fraction containing carbonylation feed and volatile promoter components which is recycled by line 12 to the carbonylation reactor 6, a high boiling base product containing carbonylation catalyst components which is recycled by line 13 to the carbonylation reactor 6, and a mixed acetic acid/acetic anhydride fraction which is withdrawn as a liquid sidestream by line 14 and passed to the separation zone 15. In zone 15 the acid/anhydride product is separated by fractional distillation into an impure acetic acid overhead fraction, which is recycled by line 16 to the esterification reactor 1, and an acetic anhydride product fraction which is withdrawn as a base product by line 17. The net acetic acid product from line 16 may be further purified is desired.
Example 1: Parts = weight or weight per hour
In this process the esterification step is carried out continuously in the kettle of a 31 plate Oldershaw column 1. Tray 16 comprises a chimney tray with liquid sidedraw. 3130 parts of methanol are fed to the kettle which contains a refluxing mixture comprising 520 parts of acetic acid, 235 parts of n-butyl acetate as internal entrainer, 10 parts of water and 40 parts of methane sulphonic acid catalyst. The temperature at tray 15 is maintained at 80 to 90°C. The sidestream from the chimney tray is cooled and separated into two phases in a decanter. The lower oil phase containing the internal entrainer is returned to tray 15. 1925 parts of aqueous phase is discharged by line 4. Recycle acetic acid (5450 parts) is fed to the kettle by line 2. A mixture of 6500 parts methyl acetate, 200 parts water and 150 parts methanol is removed as a head product by the line 5, after maintaining a 5:2 reflux to the column. The product in line 5 is made up with 220 parts of methyl acetate and 70 parts of methanol corresponding to material which could be recovered in a simple stripping stage from the waste water stream in line 4, together with 550 parts of water to give a total of 9.8% water in the feed to the carbonylation reator 6. The carbonylation reactor 6 comprises a stirred autoclave in corrosion resistant material in which the methyl acetate is reacted with carbon monoxide sparged by line 7. The reactor contains 4290 parts of a mixture containing 14 parts of rhodium expressed as rhodium diacetate, together with 40 parts of zirconyl diacetate and 215 parts of N,N'-dimethyl imidazolimium iodide. The balance comprises methyl acetate, acetic acid, acetic anhydride, and acetophenone as high boiling solvent. The carbonylation reactor 6 is maintained at a temperature of 183°C and a total pressure of 30 bar absolute. The initial catalyst components are charged by line 8, which is also used for any subsequent make-up. The product of the carbonylation reaction consisting of predominantly acetic anhydride, acetic acid, methyl iodide, solvent and unreacted methyl acetate is passed by line 10 to the separation zone 11 which is maintained at a pressure of 3 bar absolute. A high boiling base product containing all of the rhodium catalyst and zirconium and quaternary ammonium salt promoters is recycled by line 13 to the carbonylation reactor 6 together with the acetophenone and some acetic acid and acetic anhydriude. A low boiling overhead fraction containing most of the unreacted methyl acetate carbonylation feed and volatile unquaternarised methyl iodide promoter is recycled by line 12 to the carbonylation reactor 6. 10,490 parts of a mixture of acetic acid and acetic anhydride vapourised in the flash stage of separation zone 11 at a temperature of ca 135°C is recoverable as an intermediate fraction by a liquid sidestream and passed by line 14 to the separation zone 15. This comprises a 40 plate Oldershaw column with the feedpoint at plate 15 maintained at a temperature of 126°C to give a measured kettle temperature of 143°C and a heads temperature of 118°C 5030 parts of a base product are recovered by line 17 containing 98.5% of acetic anhydride. With a column reflux of 2:1 an overhead product of 5460 parts of acetic acid is obtained by line 16, containing trace levels of methyl acetate and methyl iodide. All the acetic acid is recycled to the esterification column 1 by line 2.
Example 2
The process is repeated as Example 1, save that the water in line 5 to the carbonylation stage is made up to 955 parts corresponding to 12.1% of the carbonylation feed. In this case 10,640 parts of mixed acetic acid and acetic anhydride is recovered from the separation zone 11 and fed by line 14 to separation zone 15. 3845 parts of acetic anhydride are recovered as a product by line 17 and a net acetic acid product of 1330 parts recovered by line 16 for use or further purification if required.
The above described process has the following advantages:
The acetic acid for the esterification step (or at least a significant portion thereof) is passed directly from the separation stage (in which it is separated from the acetic anhydride) without treatment. This means the esterification feed is not dependent on recovered acetic acid from subsequent operations involving the use of acetic anhydride.
The process offers flexibility in acid/anhydride product ratios without the need for a separate facility to hydrolyse anhydride to acid.
The esterification product is passed directly from the esterification reactor (from which water is removed) to the carbonylation reactor without any treatment such as purification or drying.
1) reacting methanol with recycle acetic acid in an esterification step to form an esterification product containing predominantly methyl acetate, water and optionally unreacted methanol,
2) removing part of the water from the esterification product,
3) reacting the esterification product still containing water with carbon monoxide in a carbonylation step in the presence as catalyst of free or combined metallic carbonylation catalyst and as promoter of free or combined halogen to form a carbonylation product containing acetic acid and acetic anhydride,
4) separating the carbonylation product by fractional distillation into a low boiling fraction containing carbonylation feed and volatile carbonylation promoter components, acetic acid and acetic anhydride fractions, and a higher boiling fraction containing carbonylation catalyst components,
5) recycling the low boiling fraction containing carbonylation feed and carbonylation promoter components and the higher boiling fraction containing carbonylation catalyst components to the carbonylation step and,
6) recycling at least part of the acetic acid fraction to the esterification step.
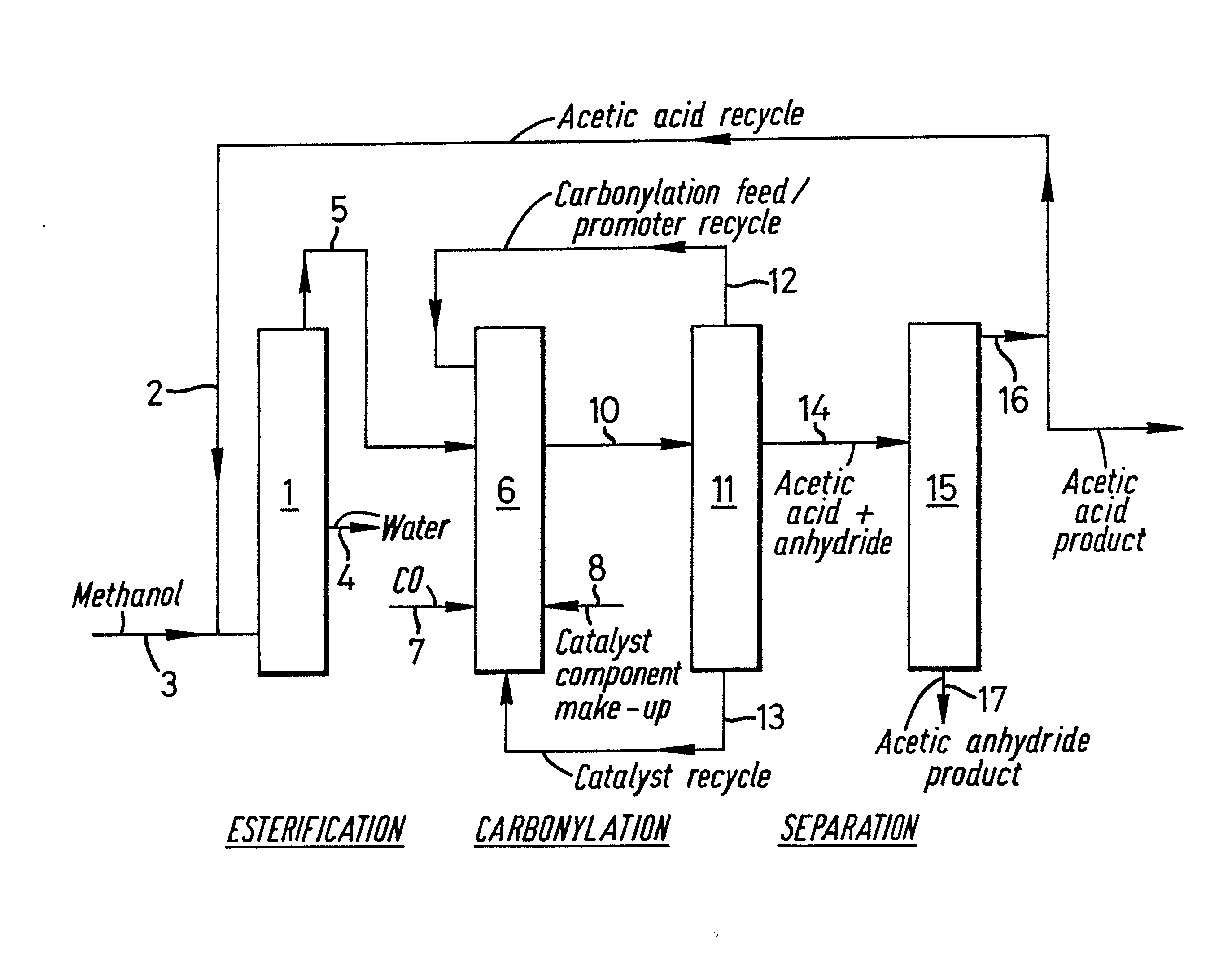
Ref : EPO - European publication server
IV- The produczion of Acetic anhydride from Vinegar and baking soda
