- Hits: 60
Sulfur Dioxide production
flow chart for sulfur dioxide production (Lab Scale)
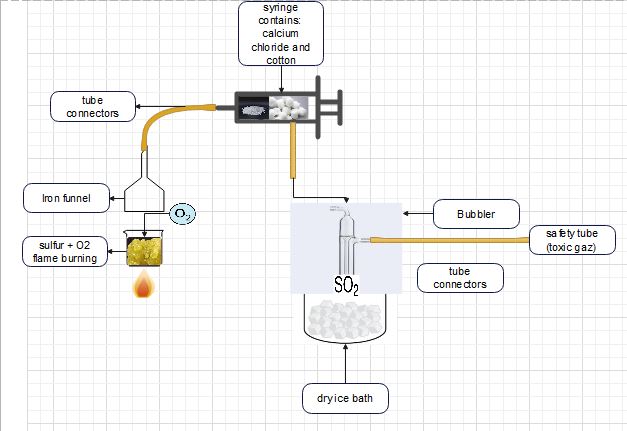
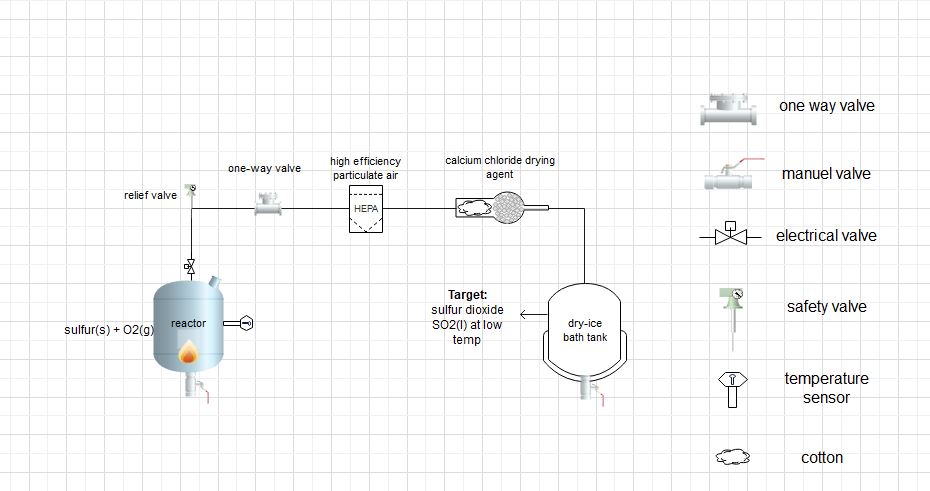
flow chart for sulfur dioxide production (Pilot scale)
Poster:
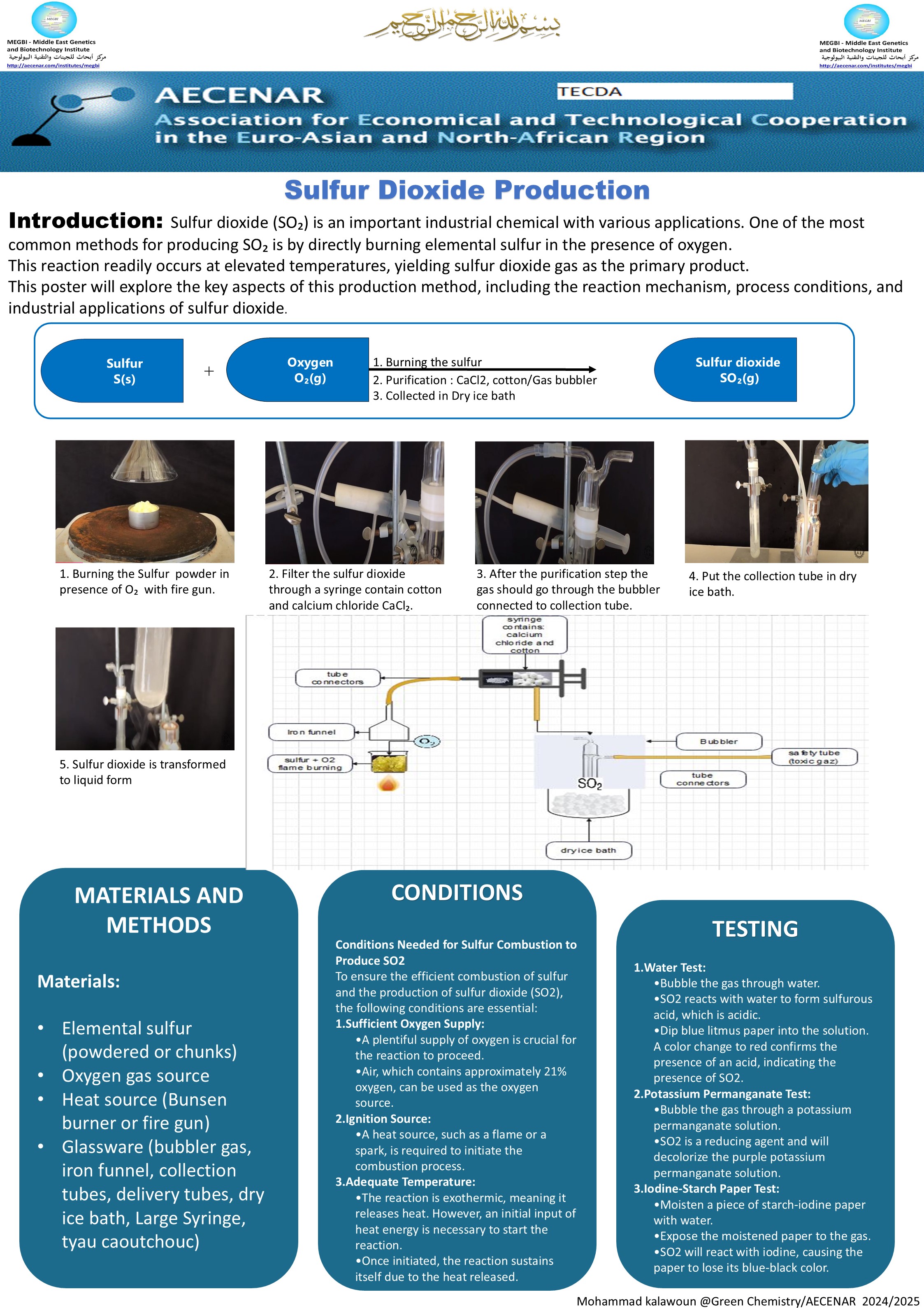
Protocol for the Production of Sulfur Dioxide (SO2) by Burning Sulfur (S) with Oxygen (O2)
Reaction:
S(s) + O2(g) → SO2(g)
Equipment:
- Combustion Chamber: A heat-resistant chamber (e.g., ceramic or stainless steel) designed for controlled combustion.
- Sulfur Feed System: A mechanism for introducing elemental sulfur into the combustion chamber (e.g., a hopper, screw feeder).
- Oxygen Supply: A source of pure oxygen or air enriched with oxygen.
- Temperature Control: A system for controlling and monitoring the reaction temperature (e.g., thermocouples, PID controller).
- Exhaust System: A system for collecting and treating the SO2 gas produced.
- Safety Equipment: Fire extinguishers, safety goggles, gloves, and a fume hood.
Procedure:
-
Set Up:
- Assemble the combustion chamber, sulfur feed system, oxygen supply, and temperature control system.
- Ensure proper ventilation and safety equipment are in place.
-
Start-Up:
- Turn on the oxygen supply and adjust the flow rate as per the desired reaction conditions.
- Ignite the sulfur feed using a suitable ignition source (e.g., a pilot flame).
-
Reaction:
- Continuously feed sulfur into the combustion chamber at a controlled rate.
- Monitor the reaction temperature and adjust the oxygen flow rate and sulfur feed rate as needed to maintain optimal conditions.
- The combustion reaction is highly exothermic, so proper temperature control is crucial to prevent overheating and potential safety hazards.
-
Product Collection:
- Collect the SO2 gas produced in a suitable collection vessel.
- The collected SO2 may require further purification depending on the intended application.
Safety Precautions:
- Toxic Gas: Sulfur dioxide is a toxic gas. Handle with care in a well-ventilated area and use appropriate personal protective equipment.
- Fire Hazard: The combustion of sulfur is highly exothermic and can lead to fires. Take necessary precautions to prevent fires and explosions.
- Emergency Procedures: Have access to emergency eyewash stations and safety showers.
Notes:
- The specific reaction conditions (temperature, pressure, flow rates) will depend on the desired production scale and purity of the SO2.
- This protocol provides a general guideline. Specific procedures and equipment may vary depending on the application and available resources.
- Always consult with qualified professionals and adhere to all relevant safety regulations when conducting chemical experiments.
This detailed protocol outlines the key steps involved in producing sulfur dioxide by burning sulfur with oxygen. Remember to prioritize safety and adhere to all necessary precautions when conducting this experiment.
